
The United States—and the world—wants to achieve carbon neutrality by the year 2050. Hydrogen (H2) is an attractive alternative energy source because of its high energy density (141.8 x 106 kJ/kg), which is three times greater than gasoline. In addition, it generates no carbon emissions when used. Thus, “green” H2 is a zero-carbon fuel created using electricity from renewable sources (e.g., solar power). With it, we get a high-density fuel that does not generate greenhouse gases, whether from its production or its consumption. Green H2 is a win-win proposition!
Innovating For A Sustainable Future
We are bringing capabilities to create the future for the scale-up of university-developed green H2 technology.
Our model, which is to develop the proof-of-concept and then follow it up with pilot- and large-scale operations, has been used successfully as exemplified by our highly praised design and operation of the radiological waste treatment processes for the Department of Energy’s (DOE’s) Salt Waste Processing Facility, in Aiken, South Carolina.
Traditional Approach To Green H2 Production – Electrolysis Of Water
Production of H2 from electrolysis of water at ambient temperature involves using electricity sourced from renewables. For this process, about 45 kWh to 55 kWh of electricity is required to produce 1 kilogram of green H2. The efficiency of the process is 60 percent, which can be improved to 70 percent with the use of catalysts.
The three water electrolysis methodologies are as follows:
- Alkaline (KOH) Electrolyzer (AE)
- Operating pressure: 1 bar to 30 bar
- Most mature, and widely used in fertilizer and chlorine industries
- Proton Exchange Membrane (PEM) Electrolyzer
- Operating pressure: 30 bar to 60 bar
- Relatively small, but can be flexible in operation
- Requires high-purity water, needs to be equipped with a deionizer
- Uses expensive catalysts (Pt, Ir) and membrane materials that have short lifetimes
- Solid Oxide (ZrO2) Electrolyzer
- High temperature (> 600 ºC) steam electrolysis, requires one-third less electricity than AE or PEM
- Uses ceramic materials
- Not commercial (but heat source from nuclear energy might make it commercially feasible)
Approaches To Green H2 Production In Research And Development Stage
There are six viable green technologies for H2 generation that are at various stages of research and development. These technologies are listed below from most to least viable.
1. Photocatalytic Water Splitting
Photocatalytic H2 production is a process that converts solar energy into chemical energy by means of a suitable photocatalyst. Dye (eosin Y)-sensitized carbon nanotubes (CNTs) and reduced graphene oxide (rGO) with a Pt cocatalyst (which stabilizes the dye) have been reported as efficient catalysts for H2 production under visible irradiation.
2. Photoelectrochemical Water Splitting
The photochemical-based direct conversion of H2O into H2 and O2 gas combines a photovoltaic system (light harvesting) and an electrolyzer (water splitting) into a single monolithic device.
3. (Biphase) Particulate Photocatalysis of Steam
The biphase particulate photocatalytic system is composed of integrated photothermal-photocatalytic materials that use charred wood substrates to convert liquid water to water steam (via photothermal transpiration effect). This steam is simultaneously split into H2 and O2 by the photocatalysts (e.g., Co or CuS/MoS2) loaded on the wood under light illumination without expending additional energy.
4. Photoreforming
The process of H2 photoproduction from a variety of organic compounds (referred to as oxygenates or sacrificial agents) like methanol, ethanol, amino acids and protein, raw biomass, aliphatic/aromatic compounds, fossil fuels, carbon monoxide, and lactic acid over TiO2 and CdS catalysts is called photoreforming.

Using methanol as the sacrificial agent:
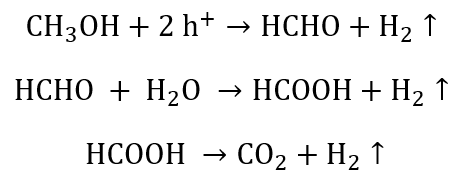
Using ethanol as the sacrificial agent:
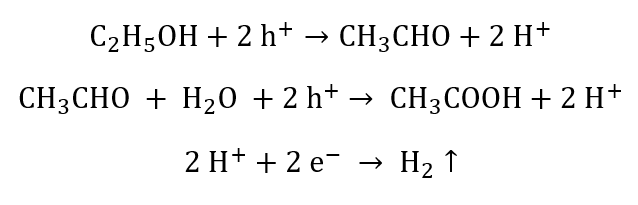
5. Using Nano-Technology Coupled With Resonance To Split Water Molecule
Two types of dissociation pathways for water molecule–resonance-enhanced vibrational excitation and electronic excitation are selectively achieved by means of injecting tunneling electrons at the single-molecule level, resulting in different dissociated products according to the following reaction paths:

Combining the above equations i. and ii., we get equation iii. as follows:
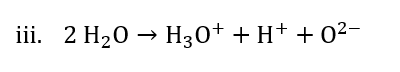
Finally, two hydrogen ions combine to make a hydrogen molecule as seen in equation iv:
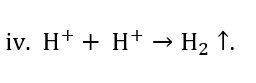
6. Simulated Photosynthesis
The first stage of (natural) photosynthesis in plants involves using sunlight to split water molecules into oxygen, hydrogen ions, and electrons. This reaction is catalyzed by a highly reactive cluster of one calcium and four manganese ions that lie at the heart of photosystem II—a complex array of proteins and other chemicals. Nature’s photosystem II is simulated by scientists whereby manganese-based catalysts surrounded by organic compounds are used to split water.
From the six processes, above, our university collaboration would formalize the technology that has the quickest potential to be developed into a viable H2 generation technology and leverage it to secure technology development funding through the formal request for proposal (RFP) process. Funding sources could be federal government (e.g., Department of Energy) or state government (i.e., California Energy Commission).
The Hydrogen Council estimates that by the year 2030, 230 TWh to 250 TWh of surplus solar and wind energy could be converted to H2. It has been reported that as much as 20 percent of the total energy consumed by the year 2050 could be from H2.
We are eager to accept the challenge of achieving a carbon-neutral future. Our successful track record of developing the proof-of-concept followed by pilot- and large-scale operations demonstrates the path from nascent technology to everyday use. This is how innovators create a better world.